Questions
frequent
Learn more about the Badge Program Quality CBR-2022

The CBR Quality Seals Program evaluates the method of performing imaging exams through information about equipment, clinical staff, images and reports. Therefore, the certification is exclusive to that evaluated modality.
In this way, the seal represents, for patients who see it on their examination report, the guarantee that the clinic/hospital was submitted to CBR evaluation, and that the institution evaluated and attested to the technical quality of the images and examination reports, considering -them suited to their rigorous standards.
Seal certification is carried out by CNPJ and by equipment, except for the Ultrasound modality, where the certificate is issued only by CNPJ.
The Quality Seals Program is aimed at public or private imaging diagnostic institutions that provide Mammography, Ultrasound, Magnetic Resonance and Computed Tomography. As long as they meet the minimum quality and safety requirements.
To enroll in the CBR Quality Seal Program, the service must pre-register through our portal. https://selos.cbr.org.br/Login.
In the Selos portal, you can pre-register, informing the requested data and attaching the necessary documents. You will then receive your login and password to use the portal.
Oh yes, you can choose which quality program you want to be part of.
It is extremely important that you carefully read all the rules of the program of interest. It contains all the prerequisites in detail, so that you can participate in the Quality Seal Program.
However, if you have already pre-registered and have not yet received your login and password, please contact us via email quality@cbr.org.br.
The criteria depend on the modality, but some criteria are the same for any program:
General criteria for all modalities:
- Payment of registration fee
- Submission of the updated Articles of Incorporation / Bylaws
- Submission of updated City Hall Permit
- Submission of updated Health Surveillance Permit with registration in a notary's office
- Submission of proof of registration of the technical manager in the updated CRM
- Submission of proof of updated Legal Entity Registration (CNPJ)
- Submission of proof of enrollment in the updated National Register of Health Establishments (CNES)
- Submission of proof of title of doctors who work in the modality to be certified (only doctors who do not have a title issued by the CBR)
- Invoice of the equipment to be certified
Mammography requirements:
- Perform the dosimeter test via CBR
- All physicians (100%) on the service must meet one of the three criteria below:
- Be a member of CBR or be an affiliate (as defined on the CBR website);
- Residency in Radiology and Diagnostic Imaging recognized by MEC.
- 75% (seventy-five percent) of the clinical staff must be up to date with the contribution to the CBR.
- In the specific case of services that use outsourced companies for the interpretation of mammography exams (teleradiology), the documentation of 100% (one hundred percent) of the company's physicians who will carry out the analysis and report of the exams must be sent. They must also have the RQE in the CRM of the state in which they are registered, as well as follow the Teleradiology Regulations (Resolution No. 2107 of 09/25/2014).
Requirements for Ultrasound:
- Doctor responsible:
- Be a Full Member of the CBR.
- Possess the Title of Specialist in Diagnostic Imaging or in General Ultrasonography (both issued by the CBR in conjunction with the AMB).
- Be up to date with CBR associative contributions.
- All doctors who work in the modality must have:
- Title of Specialist in Diagnostic Imaging with exclusive performance in General Ultrasonography, or Title of Specialist in Radiology and Diagnostic Imaging, or certificate in the area of expertise granted or recognized by CBR/AMB, or Medical Residency in Radiology and Diagnostic Imaging recognized by MEC .
- Foreign physicians must have a diploma validated by the Federal Council of Medicine (CFM) and evidence that enables them to carry out activities in the field of ultrasound.
- 75% of the clinical staff must be up to date with the associative contribution with the CBR, including the responsible physician.
Requirements for Tomography and MRI:
- The physician responsible for the service must have the following qualifications:
- Be a Full Member of the CBR.
- Possess the Title of Specialist in Radiology and Diagnostic Imaging.
- Be up to date with CBR associative contributions.
- 75% (seventy-five percent) of the clinical staff must be up to date with the contribution to the CBR.
- All physicians in the service must be a full member of the CBR or be affiliated (as defined on the CBR website) or have residency in Radiology and Diagnostic Imaging recognized by the MEC.
- In the specific case of services that use outsourced companies for the interpretation of MRI exams (teleradiology), the documentation of 100% of the doctors of the company that will carry out the analysis and report of the exams must be sent, and these must comply with item 2.1.
The main feature of the CBR Quality Seal Program is technical evaluation, focusing on image evaluation and the report of the respective method.
Padi, in addition to the technical assessment, has a broader scope that includes the on-site audit that assesses the entire service delivery management chain.
The values are annual and updated according to the current year. To consult the current value, consult the regulations of interest at: https://selos.cbr.org.br/Login.
Remembering that the values are the same for all certified modalities.
Services associated with ABCDI and/or PADI accredited have 10% off the annual fee of the Quality Seal Program. This discount does not apply to stamp purchases.
To participate in the Quality Seals Program, the clinic does not need to be associated with the CBR. However, 75% of physicians who work in the modality to be certified in the clinic, must be associated with and in compliance with the CBR.
In the case of Mammography, the physicians' 75% must be up to date with the associative contribution from CBR or SBM or Febrasgo.
Yes, in the specific case of services that use outsourced companies for the interpretation of the exams (teleradiology), the documentation of 100% (one hundred percent) of the doctors of the company that will carry out the analysis and report of the exams must be sent, and these must meet the requirement of 100% of the members of the service being specialists in Radiology and Diagnostic Imaging or possessing a certificate in the area of expertise conferred by the CBR. They must also have the RQE in the CRM of the state in which they are registered, as well as follow the Teleradiology Regulations (Resolution No. 2107 of 09/25/2014).
In addition, the observation for teleradiology services in the responsible physician and clinical staff items of the specific Regulation must be checked.
If you do not perform all the exams described in the regulations of the desired program, the service must consult the CBR team before enrolling, for an assessment of whether these pending issues may imply participation in the program.
As stated in the respective regulations, the injection pump is mandatory equipment, therefore it is not possible to enroll in the stamp programs of these modalities without this equipment.
Document renewal protocols will be accepted for enrollment in the program, as long as they are up to date. However, the official documents must be presented until the end of the process for the release of the certificate. In cases of initial request protocol, that is, the definitive document does not yet exist, we advise you to wait for its release, before starting the Quality Seal Program process.
Yes, the dosimeter testing phase is a mandatory phase for all mammography services that participate in the CBR Quality Seal Program in this modality. If you do not have the X-ray breast simulator (phantom breast) for these tests, the CBR may make it available on loan. To do so, we advise you to consult the CBR Quality team on the phone (11) 3372-4550 or by e-mail at quali@cbr.org.br, for clarification on this procedure.
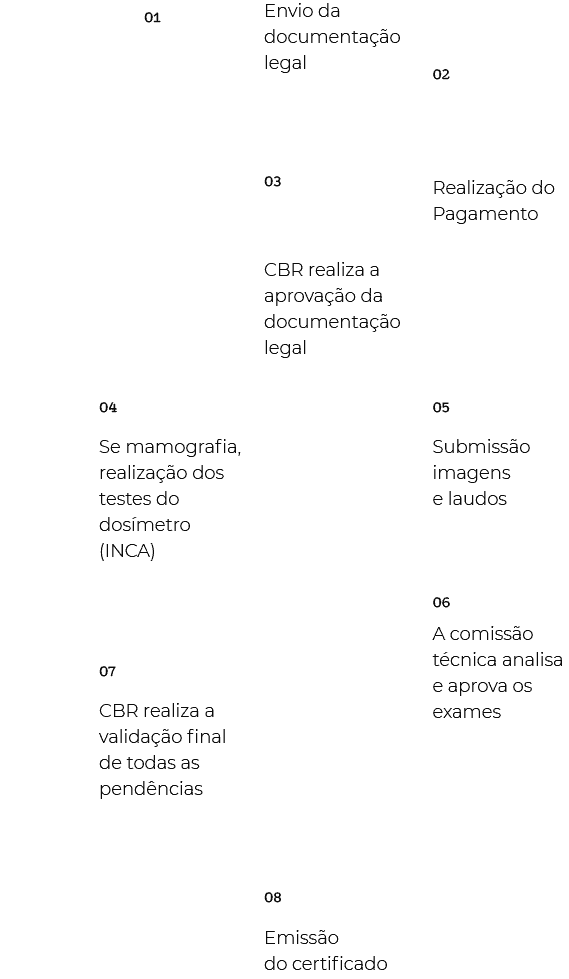
Exams are sent electronically through a platform parallel to the program's process monitoring system. After approval of the legal documentation, the service will receive an access link followed by all the guidelines to send the exams with the respective reports for evaluation. Remembering that the exams sent must have been performed within a maximum of 90 days from the date of submission, and must present pathology (described in regulations).
All exams must be in DICOM format, and the reports in PDF with duly anonymized data (name, address, telephone, letterhead, clinic watermark, patient's name, doctor's name, medical insurance, CRM, signature of the doctor, header with identification) or any other information that allows identifying the service, the patient or the doctor.
To find out which exams should be sent, consult the regulations of the program of interest. There you will find the list of exams, and the protocols to be followed.
Only Ultrasound programs can send images in DICOM or JPEG format.
Having as a differential the technical evaluations of images and reports, the CBR has the participation of committees of radiologists whose main role is to provide programs generated and controlled by the class itself, at accessible costs, through the evaluation of exams of the participating services and the generation of technical contents that improve the practice of the specialty.
In each of the areas that make up the CBR Quality Seals Program, there are committees composed of several specialized radiologists. The groups operate autonomously, impartially and inclusively, without punitive characteristics and are composed of representative members with extensive experience in the respective method.
The main role of the CBR Commissions is to provide programs generated and controlled by the class itself, at affordable costs, a factor that has contributed over time to preventing the emergence of parallel companies to explore this segment.
All requests related to enrollment in the Quality Seal Program and purchase of seals must be made through the Seal Portal system https://selos.cbr.org.br/Login.
There you can follow your entire process, revisions, revalidations, purchase of stamps, and possible issues.
Pending issues are highlighted in the "Home" when accessing, and by clicking on each task, it is possible to proceed with the requests. See “Tutorial for using Portal Selos”.
The change of any registration data must be requested to the CBR team through the e-mail quality@cbr.org.br or by telephone: 11 3372-4550.
The replacement of incorrect or expired documents (attachments) can be carried out at any time in the Stamp Portal > Company > Company Data > Documents > “Edit” button. See “Tutorial for using Portal Selos”.
You can recover your access password through the “Recover Password” button by entering your user email. If you do not remember, or do not have the access email anymore, please contact the CBR team, so that we can grant a new access, after confirming the data.
In order to insert a new user in the Portal Selos system, the “main user” must register the data of this new user in the menu Users > New User. see “Tutorial for using Portal Selos”.
Quality seals in adhesive label format, as well as electronic art that can be used in disclosures and communications about service certification in the Quality Seal Programs,
they can only be acquired after approval and during the term of the program.
Yes, the manual for using the electronic art is sent by email to each service, along with the customized files for use. See visual identity manual through the link:
All requests related to the purchase of stamps must be made through the Portal Selos system, with your username and password through the section “Request for the purchase of Stamps” on the home page.
By clicking on the “shopping cart” icon, which is only displayed when the stamp program is active.
Purchases are always made by the thousand, for example: for orders of 1000 stamps, simply fill in the number “1” in the field “Amount of Stamps Desired”. Just below the item “Purchase Value” appears the amount to be paid. The follow-up of this order, as well as the release of payment, takes place in this same section. If the request is denied by the CBR Quality team, the denial will appear
in the “Pending” section. See “Tutorial for using Portal Selos”.
All invoices are issued to the services at the end of the month in which the payment was made. If the service requires the invoice beforehand, we advise you to request it from the finance department via email
financial@cbr.org.br.
The query must be made through the Selos Portal with your username and password, accessing the “Quality Seal Programs” area, you will be able to consult the situation of your program (status).
We advise you to start revisions/revalidations 3 months before the expiration of the program, thus having enough time to make the necessary adjustments. In case of doubt, consult the CBR Quality team.
The annual review is maintenance of your certified program. When the service is approved in the Quality program, its certification is valid for 3 years, and on each anniversary date (day/month of the approval date), maintenance is required.
This maintenance consists of updating expired documents, registration data, equipment and clinical staff information, if necessary, and paying the fee.
Revalidation, on the other hand, is when the three-year period (3 years) of your program expires. In this case, in addition to updating documents, equipment, clinical staff and payment of the fee, the image evaluation phase and dosimeter tests (only for mammography programs) are required, so that it can be certified again for another 3 years.
Both revision and revalidation must be requested by the certified service through the Portal Selos > Home > Pending issues system.
Updating equipment data is very important in the quality seal program.
When exchanges or inclusion of new equipment occur, or change of service address, the current certification is terminated and the process must start again, since the
Quality Seals, certify the service equipment. When there is an exchange or inclusion of new devices, it is necessary to start the process again to evaluate the new devices.
The request must be made by email to quali@cbr.org.br, informing the following data: company name, CNPJ, address, type of certificate, name of the physician responsible for the service and reason for reissuing the certificate. There is a charge for issuing the 2nd copy of the certificate in the amount of R$200.00. We emphasize that we do not reissue certificates with a retroactive expiration date, only for current programs.
The list of approved services is available on the CBR website at: https://cbr.org.br/cbr-clinicas/.
The Norms and Regulations are documents with technical guidelines and rules about the Quality Seal Program.
Read the regulations for the modality desired for certification (Mammography, Resonance, Tomography, Ultrasound) on the Norms and Labels page (https://cbr.org.br/normas-selos/).
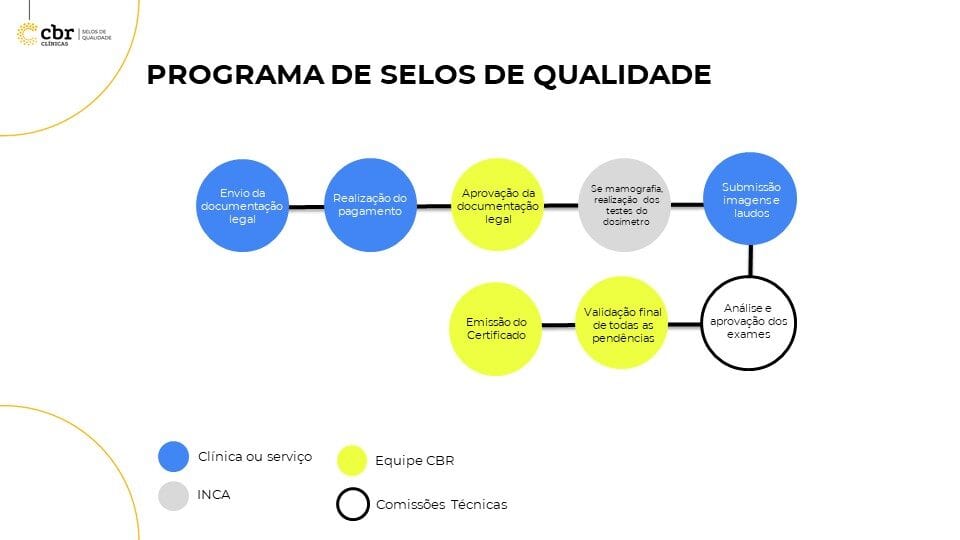
HOW THE CERTIFICATION PROCESS WORKS